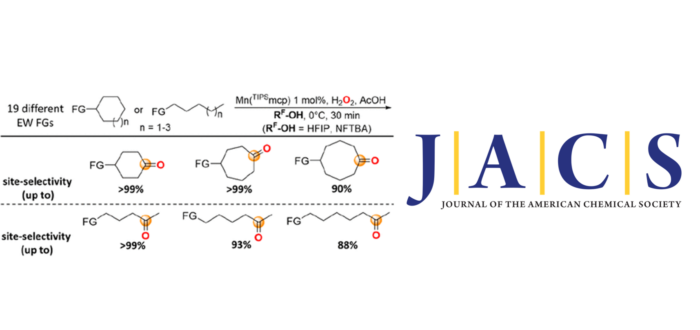
A detailed study on the C(sp3)–H bond oxygenation reactions with H2O2 catalyzed by the [Mn(OTf)2(TIPSmcp)] complex at methylenic sites of cycloalkyl and 1-alkyl substrates bearing 19 different electron-withdrawing functional groups (EW FGs) was carried out. Oxidations in MeCN were compared to the corresponding ones in the strong hydrogen bond donating (HBD) solvents 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) and nonafluoro tert-butyl alcohol (NFTBA). Formation of the products deriving from oxygenation at the most remote methylenic sites was observed, with yields, product ratios (PR) for oxygenation at the most remote over the next methylenic sites, and associated site-selectivities that significantly increased going from MeCN to HFIP and NFTBA. Unprecedented site-selectivities were obtained in the oxidation of cyclohexyl, cycloheptyl, cyclooctyl, 1-pentyl, 1-hexyl, and 1-heptyl substrates, approaching >99%, >99%, 90%, >99%, 93%, and 88% (PR >99, >99, 9.4, >99, 14, and 7.5) with cyclohexyl-2-pyridinecarboxylate, cycloheptyl-2-pyridinecarboxylate, cyclooctyl-4-nitrobenzenesulfonamide, 1-pentyl-3,5-dinitrobenzoate, 1-hexyl-3,5-dinitrobenzoate, and 1-heptyl-3,5-dinitrobenzoate, respectively. The results are rationalized on the basis of a polarity enhancement effect via synergistic electronic deactivation of proximal methylenic sites imparted by the EWG coupled to solvent HB. Compared to previous procedures, polarity enhancement provides the opportunity to tune site-selectivity among multiple methylenes in different substrate classes, extending the strong electronic deactivation determined by native EWGs by two carbon atoms. This study uncovers a simple procedure for predictable, high-yielding, and highly site-selective oxidation at remote methylenes of cycloalkyl and 1-alkyl substrates that occurs under mild conditions, with a large substrate scope, providing an extremely powerful tool to be implemented in synthetically useful procedures.
This work has been performed in collaboration of Prof. Massimo Bietti (Università “Tor Vergata”, Italy), and Dr. Marco Galeotti, Filippo Scarchilli, Prof. Miquel Costas of the QBIS-CAT group of the Institute of Computational Chemistry and Catalysis (IQCC) of the University of Girona.
It has been recently published open access in Journal of the American Chemical Society:
S. Sisti, M. Galeotti, F. Scarchilli, M. Salamone, M. Costas*, and M. Bietti*
“Highly Selective C(sp3)–H Bond Oxygenation at Remote Methylenic Sites Enabled by Polarity Enhancement.“
J. Am. Chem. Soc., 2023, ASAP
DOI: 10.1021/jacs.3c07658
Girona, October 6, 2023
For more info: ges.iqcc@udg.edu