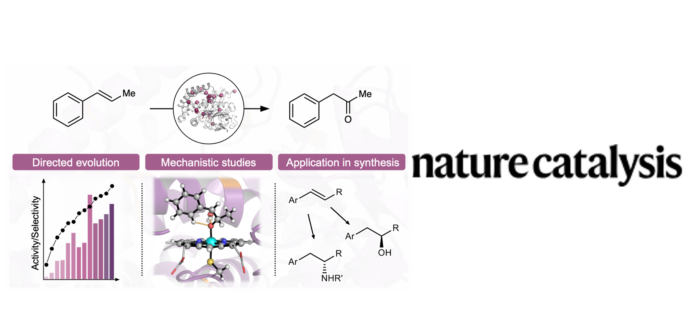
Ketones are crucial intermediates in synthesis and frequent moieties in many products. The direct regioselective synthesis of ketones from internal alkenes could simplify synthetic routes and solve a long-standing challenge in catalysis.
In this work, the groups of Prof. Stephan Hammer (University of Bielefeld, Germany) and Dr. Marc Garcia-Borràs (IQCC), report the laboratory evolution of a cytochrome P450 enzyme for the direct oxidation of internal arylalkenes to ketones with several thousand turnovers. This evolved ketone synthase benefits from 15 crucial mutations, most of them distal to the active site. Computational analysis revealed that all these mutations collaborate to generate and tame a highly reactive carbocation intermediate. Engineered enzyme active sites can exert an exceptional degree of control over reactive intermediates. This is achieved in the KS evolution by generating a confined, rigid and preorganized active site through multiple mutations that enhance a pre-existing dynamic network, which geometrically and electrostatically preorganize the active site to provides access to a highly reactive carbocation species and thus enables direct ketone production from high-valent metal–oxo species.
The engineered enzyme exploits a metal–oxo species for ketone synthesis and enables various challenging alkene functionalization reactions. This includes the catalytic, enantioselective oxidation of internal alkenes to ketones and formal asymmetric hydrofunctionalizations of internal alkenes in combination with other biocatalysts.
These findings demonstrate that cytochrome P450 can be evolved to oxidize internal arylalkenes to ketones with high activity and selectivity. The authors used the evolved KS to directly convert internal arylalkenes into ketones, including a catalytic enantioselective example. In addition, the KS is combined with established biocatalysts to convert internal arylalkenes into chiral phenylethanols and phenylethylamines on a preparative scale with high activity, regio- and enantioselectivity. What stands out is that the KS enables many important, highly selective functionalization reactions of internal arylalkenes that have so far largely eluded efficient and selective catalysis.
Based on their results, the authors foresee that the high degree of confinement in enzyme active sites could be generally leveraged to enable other challenging catalytic cycles by precisely controlling the accessible conformations of reactive intermediates. The latter is, indeed, the main research line of Dr. Garcia-Borràs and his group.
This collaborative work has been carried out by Sebastian Gergel (Hammer lab) and Jordi Soler (Garcia-Borràs Lab) as co-first authors, Alina Klein and Lai Schülke (Hammer lab), Bernhard Hauer (University of Stuttgart), under the guidance of Stephan Hammer (University of Bielefeld) and Marc Garcia Borras (IQCC). The work has been recently published in Nature Catalysis:
S. Gergel, J. Soler, A. Klein, K. H. Schülke, B. Hauer, M. Garcia-Borràs* and S. C. Hammer*
“Engineered cytochrome P450 for direct arylalkene-to-ketone oxidation via highly reactive carbocation intermediates”
Nat. Catal. 2023, ASAP
DOI: 10.1038/s41929-023-00979-4
Girona, July 20, 2023
For more info: ges.iqcc@udg.edu