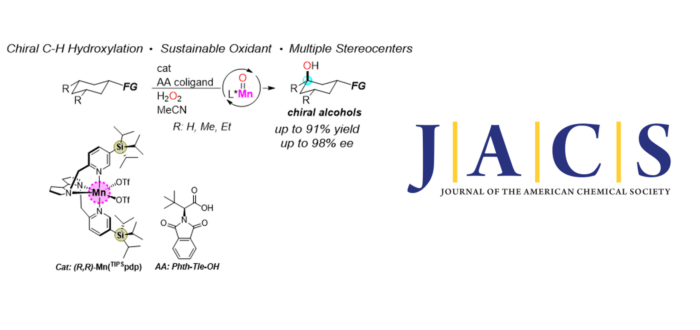
The development of novel strategies to introduce chirality at a molecular level is a longstanding pursuit within the organic chemistry community. The generation of chiral alcohols has an important role for the synthesis of many bioactive molecules, however the asymmetric construction of C-O in an enantioselective manner from C(sp3)-H bond rich and readily available starting materials, represents a major challenge for organic chemists. In this study, Andrea Palone, Guillem Casadevall, Sergi Ruiz-Barragan, Arnau Call, Sílvia Osuna, Massimo Bietti, and Miquel Costas present the first example of non-directed and non-enzymatic catalytic enantioselective hydroxylation of unactivated tertiary C(sp3)-H bonds. Theoretical analysis of the origin of the enantioselectivity uncovers that chiral recognition relies in a synergistic interplay of weak interactions and structural complementary between substrate and catalyst that resemble lock-and-key recognition phenomena operating in enzymatic sites. Furthermore, the use of catalysts based on an earth abundant metal singularizes the current system with respect to state-of-the-art asymmetric C(sp3)-H functionalization reactions that rely on precious metals. In addition, the use of hydrogen peroxide as oxidant confers the system a high atom economy, which combined with the mild experimental conditions makes the reaction particularly appealing from a sustainability perspective. Stereoretentive C(sp3)-H hydroxylation results in a single step generation of multiple stereogenic centers (up to four) that can be orthogonally manipulated by conventional methods providing rapid access, from a single precursor, to a variety of chiral scaffolds. Collectively, the work delivers proof of concept of the powerful reach of biomimetic oxidation catalysts as unique tools for manipulating C-H bonds as functional groups, valorizing simple organic molecules by means of their conversion into chiral, stereochemically rich and versatile oxidized products, expanding in a virtually unlimited manner the available chiral pool. Finally, the high structural versatility of the catalytic system is a key aspect that enables rapid evolution of the reaction from modest to outstanding yields and enantioselectivities. We foresee that such versatility will find utility in the development of novel enantioselective C-H functionalization reactions.
This work involved Prof. Massimo Bietti of the University Tor Vergata, and Andrea Palone, Guillem Casadevall, Dr. Sergi Ruiz-Barragan, Dr. Arnau Call, Prof. Sílvia Osuna and Prof. Miquel Costas of the QBIS-CAT group and TCBioSys of the Institute of Computational Chemistry and Catalysis (IQCC) of the University of Girona.
The study was recently published open access in Journal of the American Chemical Society:
A.Palone, G. Casadevall, S. Ruiz-Barragan, A. Call, S. Osuna*, M. Bietti* and M. Costas*.
“C–H Bonds as Functional Groups: Simultaneous Generation of Multiple Stereocenters by Enantioselective Hydroxylation at Unactivated Tertiary C–H Bonds”.
J. Am. Chem. Soc..,2023, 145, 15742-15753
DOI: 10.1021/jacs.2c10148
Girona, July 28, 2023
For more info: ges.iqcc@udg.edu