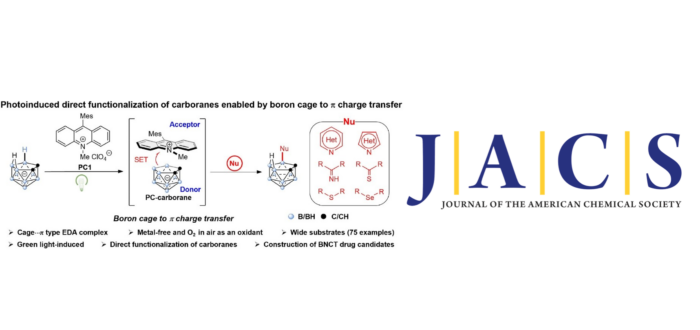
The development of new synthetic methods for B–H bond activation has been an important research area in boron cluster chemistry, which may provide opportunities to broaden the application scope of boron clusters. Herein, we present a new reaction strategy for the direct site-selective B–H functionalization of nido-carboranes initiated by photoinduced cage activation via a noncovalent cage···? interaction. As a result, the nido-carborane cage radical is generated through a single electron transfer from the 3D nido-carborane cage to a 2D photocatalyst upon irradiation with green light. The resulting transient nido-carborane cage radical could be directly probed by an advanced time-resolved EPR technique. In air, the subsequent transformations of the active nido-carborane cage radical have led to efficient and selective B–N, B–S, and B–Se couplings in the presence of N-heterocycles, imines, thioethers, thioamides, and selenium ethers. This protocol also facilitates both the late-stage modification of drugs and the synthesis of nido-carborane-based drug candidates for boron neutron capture therapy (BNCT).
This work has been carried out by S. Xu, H. Zhang, J. Xu, C.-S. Lu, D. Tu and Prof. H. Yan from Nanjing University, W. Suo and Prof. X. Guo from Tsinghua University, and Prof. Jordi Poater from the University of Barcelona and Prof. Miquel Solà from the DiMoCat group of the Institute of Computational Chemistry and Catalysis of the University of Girona.
It has been recently published open access in Journal of the American Chemical Society:
S. Xu, H. Zhang, J. Xu, W. Suo, C.-S. Lu, D. Tu*, X. Guo*, J. Poater*, M. Solà* and H. Yan*.
“Photoinduced Selective B–H Activation of nido-Carboranes”
J. Am. Chem. Soc., 2024, 146, 7791-7802
DOI: 10.1021/jacs.4c00550.
Girona, April 2nd, 2024
For more info: ges.iqcc@udg.edu