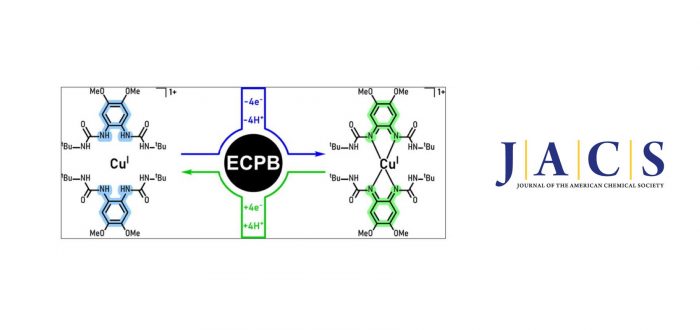
In this research article, we describe a 4H+/4e– electron-coupled-proton buffer (ECPB) based on Cu and a redox-active ligand. The protonated/reduced ECPB (complex 1: [Cu(8H+/14e–)]1+), consisting of CuI with 2 equiv of the ligand (catLH4: 1,1?-(4,5-dimethoxy-1,2-phenylene)bis(3-(tert-butyl)urea)), reacted with H+/e– acceptors such as O2 to generate the deprotonated/oxidized ECPB. The resulting compound, (complex 5: [Cu(4H+/10e–)]1+), was characterized by X-ray diffraction analysis, nuclear magnetic resonance (1H-NMR), and density functional theory, and it is electronically described as a cuprous bis(benzoquinonediimine) species. The stoichiometric 4H+/4e– reduction of 5 was carried out with H+/e– donors to generate 1 (CuI and 2 equiv of catLH4) and the corresponding oxidation products. The 1/5 ECPB system catalyzed the 4H+/4e– reduction of O2 to H2O and the dehydrogenation of organic substrates in a decoupled (oxidations and reductions are separated in time and space) and a coupled fashion (oxidations and reductions coincide in time and space). Mechanistic analysis revealed that upon reductive protonation of 5 and oxidative deprotonation of 1, fast disproportionation reactions regenerate complexes 5 and 1 in a stoichiometric fashion to maintain the ECPB equilibrium.
This work has been performed by Marcel Swart in collaboration with Michael P. Hendrich and Isaac Garcia-Bosch (Carnegie Mellon University, United States), and has recently been published in Journal of the American Chemical Society:
T. Wu, K. Rajabimoghadam, A. Puri, D. D. Hebert, Y. L. Qiu, S. Eichelberger, M. A. Siegler, M. Swart, M. P. Hendrich, and I. Garcia-Bosch
“A 4H+/4e– Electron-Coupled-Proton Buffer Based on a Mononuclear Cu Complex”
J. Am. Chem. Soc. 2022, ASAP
DOI: 10.1021/jacs.2c05454
Girona, Sep. 20, 2022
For more info: gestor.iqcc@gmail.com