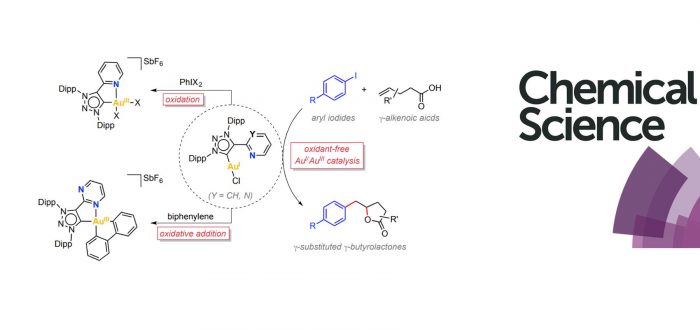
Oxidant-free Au-catalyzed reactions are emerging as a new synthetic tool for innovative organic transformations. Still, a deeper mechanistic understanding is needed for a rational design of these processes. Here we describe the synthesis of two Au(I) complexes bearing bidentated hemilabile MICˆN ligands, [AuI(MICˆN)Cl], and their ability to stabilize square-planar Au(III) species (MIC = mesoionic carbene). The presence of the hemilabile N-ligand contributed to stabilize the ensuing Au(III) species acting as a five-membered ring chelate upon its coordination to the metal center. The Au(III) complexes can be obtained either by using external oxidants or, alternatively, by means of feasible oxidative addition with strained biphenylene Csp2-Csp2 bonds as well as with aryl iodides. Based on the fundamental knowledge gained on the redox properties on these Au(I)/Au(III) systems, we successfully develop a novel Au(I)-catalytic procedure for the synthesis of a ?-substituted ?-butyrolactones through the 1,2-arylation – lactonization reaction of the corresponding ?-alkenoic acid. The oxidative addition of the aryl iodide, which is in turn allowed by the hemilabile nature of the MICˆN ligand, is an essential step of this transformation.
This work has been performed by Pau Font, Hugo Valdes and Xavi Ribas in collaboration with Gregorio Guisado-Barrios (University of Zaragoza, ISQCH), and has recently been published in Chemical Science:
P. Font, H. Valdes, G. Guisado-Barrios and X. Ribas
“Hemilabile MIC^N ligands allow oxidant-free Au(I)/Au(III) arylation-lactonization of ?-alkenoic acids”
Chem. Sci. 2022, 13, ASAP-
DOI: 10.1039/D2SC01966C
Girona, July 26, 2022
For more info: gestor.iqcc@gmail.com