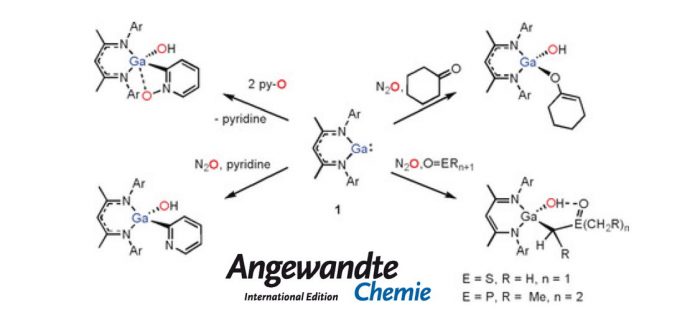
In situ oxidation of the GaI compound NacNacGa by either N2O or pyridine oxide results in the generation of a labile monomeric oxide, NacNacGa(O), which can easily cleave the C?H bonds of aliphatic and aromatic substrates featuring good donor sites. The products of this reaction are gallium organyl hydroxides. DFT calculations show that these reactions start with the formation of NacNac?Ga(O)(L) adducts, the oxo ligand of which can easily abstract protons from nearby C?H bonds, even for sp2?hybridized carbon centers. Aliphatic amines do not enter this reaction for kinetic reasons, presumably because of the unfavorable sterics.
The paper was recently published in Angewandte Chemie International Edition:
A. Kassymbek, S.F. Vyboishchikov, B.M. Gabidullin, D. Spasyuk, M. Pilkington, and G.I. Nikonov
“Sequential Oxidation and C?H Bond Activation at a Gallium(I) Center”
Angew. Chem. Int. Ed. 2019, 58, 18102-18107 [abstract]
DOI: 10.1002/anie.201913028
- sec.iqcc@udg.edu
- +34 972 41 83 57