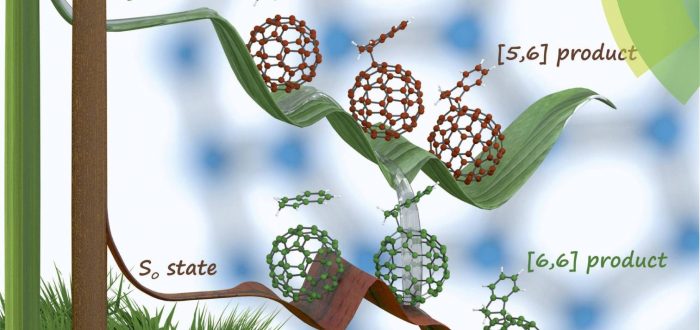
A group of chemists from the Universidad Complutense de Madrid lead by Prof. Nazario Martín together with the IQCC members Ouissam El Bakouri (ex-member of the IQCC, now in Uppsala), Miquel Solà, and Marc Garcia-Borràs (ex-member of the IQCC, now in UCLA) have studied the regioselectivity of the Diels-Alder reaction to C60in the singlet ground state and excited high spin states. DFT calculations show that the regioselectivity of the Diels–Alder (DA) cycloaddition of cyclopentadiene to 2S+1C60 changes from the usual [6,6] addition in the singlet ground state to the [5,6] attack in high spin states of C60. Changes in the aromaticity of the five- and six-membered rings when going from singlet to high spin C60provide a rationale to understand this regioselectivity change. Experimentally, however, we find that the DA cycloaddition of isoindene to triplet C60 yields the usual [6,6] adduct. Further DFT calculations and computational analysis give an explanation to this unanticipated experimental result by showing the presence of an intersystem crossing close to the formed triplet biradical intermediate.
The paper was published today in Phys. Chem. Chem. Phys.:
O. El Bakouri, M. Garcia-Borràs, R.M. Girón, S. Filippone, N. Martín, and M. Solà
“On the regioselectivity of the Diels–Alder cycloaddition to C60 in high spin states”
Phys. Chem. Chem. Phys. 2018, 20, 11577-11585 [abstract]
DOI: 10.1039/C7CP07965F
The inside front cover of the issue was dedicated to the article as well.