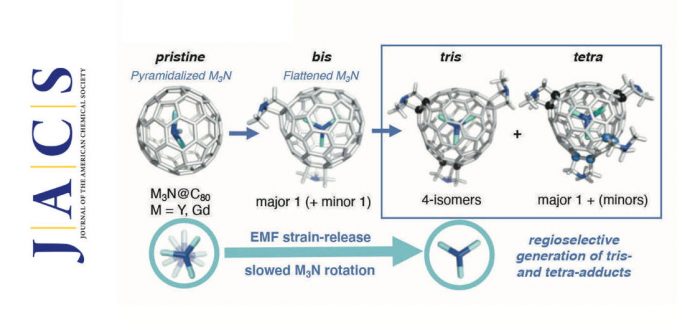
In this collaboration project between ETH Zurich, Purdue University Fort Wayne, and the IQCC (Dr. Adrià Romero-Rivera, Dr. Marc Garcia-Borràs, and Prof. Sílvia Osuna) the tris- and tetra-adducts of M3N@Ih–C80 metallofullerenes were synthesized and characterized for the first time.
The 1,3-dipolar cycloaddition (Prato reaction) of Y3N@Ih-C80 and Gd3N@Ih-C80 with an excess of N-ethylglycine and formaldehyde provided tris- and tetra- fulleropyrrolidine adducts in a regioselective manner. Purification by HPLC and analyses of the isolated peaks, by NMR, MS, and vis-NIR spectra revealed that the major products were four tris- and one tetra-isomers for both Y3N@Ih-C80 and Gd3N@Ih-C80. Considering the large number of possible isomers (e.g. at least 1140 isomers for the tris adduct), the limited number of obtained isomers indicated that the reactions proceeded with high regioselectivity. NMR analyses of the Y3N@Ih-C80 adducts found that the tris adducts were all-[6,6]- or [6,6][6,6][5,6]- isomers and that some showed mutual isomerization or remained intact at room temperature. The tetra-adduct obtained as a major product was all-[6,6] and stable.
For the structural elucidation of Gd3N@Ih-C80 tris- and tetra-adducts, DFT calculations were performed to estimate the relative stabilities of tris- and tetra-adducts formed upon Prato functionalization of the most pyramidalized regions of the fullerene structure. The most stable structures corresponded to additions on the most pyramidalized (i.e. strained) bonds. Taking together the experimental vis- NIR spectra, NMR assignments, and the computed relative DFT stabilities of the potential tris and tetra-adducts, the structures of the isolated adducts were elucidated. ESR measurements of pristine and bis-, tris-adducts of Gd3N@C80 suggested that the rotation of the endohedral metal cluster slowed upon increase of the addition numbers to C80 cage, which is favored for accommodating the Gd atoms of the relatively large Gd3N cluster inner space at the sp3 addition sites. This is presumably related to the high regioselectivity in the Prato addition reaction driven by the strain release of the Gd3N@C80 fullerene structure.
The results were reported recently in the Journal of the American Chemical Society:
O. Semivrazhskaya, S. Aroua, M. Yulikov, A. Romero-Rivera, S. Stevenson, M. Garcia-Borràs, S. Osuna, and Y. Yamakoshi
“Regioselective Synthesis and Characterization of tris- and tetra-Prato Adducts of M3N@C80 (M = Y, Gd)”
J. Am. Chem. Soc. 2020, [], ASAP-
DOI: 10.1021/jacs.9b13768
Girona, July 2, 2020
For more info: sec.iqcc@udg.edu