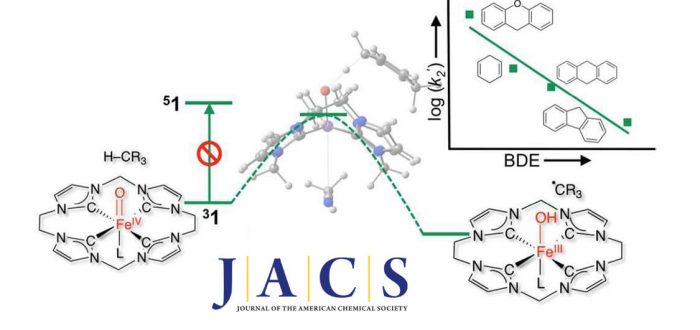
C–H bond activation mediated by oxo-iron (IV) species represents the key step of many heme and nonheme O2-activating enzymes. Of crucial interest is the effect of spin state of the FeIV(O) unit. Here we report the C–H activation kinetics and corresponding theoretical investigations of an exclusive tetracarbene ligated oxo-iron(IV) complex, [LNHCFeIV(O)(MeCN)]2+ (1). Kinetic traces using substrates with bond dissociation energies (BDEs) up to 80 kcal mol–1 show pseudo-first-order behavior and large but temperature-dependent kinetic isotope effects (KIE 32 at ?40 °C). When compared with a topologically related oxo-iron(IV) complex bearing an equatorial N-donor ligand, [LTMCFeIV(O)(MeCN)]2+ (A), the tetracarbene complex 1 is significantly more reactive with second order rate constants k’2 that are 2–3 orders of magnitude higher. UV–vis experiments in tandem with cryospray mass spectrometry evidence that the reaction occurs via formation of a hydroxo-iron(III) complex (4) after the initial H atom transfer (HAT). An extensive computational study using a wave function based multireference approach, viz. complete active space self-consistent field (CASSCF) followed by N-electron valence perturbation theory up to second order (NEVPT2), provided insight into the HAT trajectories of 1 and A. Calculated free energy barriers for 1 reasonably agree with experimental values. Because the strongly donating equatorial tetracarbene pushes the Fe-dx2–y2 orbital above dz2, 1 features a dramatically large quintet-triplet gap of ?18 kcal/mol compared to ?2–3 kcal/mol computed for A. Consequently, the HAT process performed by 1 occurs on the triplet surface only, in contrast to complex A reported to feature two-state-reactivity with contributions from both triplet and quintet states. Despite this, the reactive FeIV(O) units in 1 and A undergo the same electronic-structure changes during HAT. Thus, the unique complex 1 represents a pure “triplet-only” ferryl model. The results were published today in Journal of the American Chemical Society (J. Am. Chem. Soc. 2017, ASAP):
C. Kupper, B. Mondal, J. Serrano-Plana, I. Klawitter, F. Neese, M. Costas, S. Ye, and F. Meyer
“Nonclassical Single-State Reactivity of an Oxo-Iron(IV) Complex Confined to Triplet Pathways”
J. Am. Chem. Soc. 2017, ASAP [abstract]
DOI: 10.1021/jacs.7b03255
- sec.iqcc@udg.edu
- +34 972 41 83 57