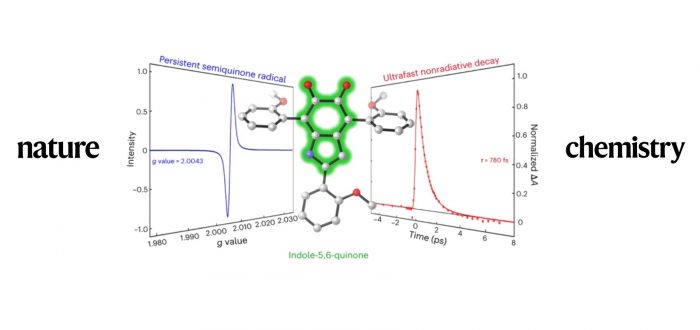
Melanins are ubiquitous biopolymers produced from phenols and catechols by oxidation. They provide photoprotection, pigmentation and redox activity to most life forms, and inspire synthetic materials with desirable optical, electronic and mechanical properties. The chemical structures of melanins remain elusive, however, creating uncertainty about their roles, and preventing the design of synthetic mimics with tailored properties. Indole-5,6-quinone (IQ) has been implicated as a biosynthetic intermediate and structural subunit of mammalian eumelanin pigments, but its instability has prevented its isolation and unambiguous characterization. Here we use steric shielding to stabilize IQ and show that ‘blocked’ derivatives exhibit eumelanin’s characteristic ultrafast nonradiative decay and its ability to absorb light from the ultraviolet to the near-infrared. These new compounds are also redox-active and a source of paramagnetism, emulating eumelanin’s unique electronic properties, which include persistent radicals. Blocked IQs are atomistically precise and tailorable molecules that can offer a bottom–up understanding of emergent properties in eumelanin and have the potential to advance the rational design of melanin-inspired materials.
This work has been carried out byL. Kinziabulatova, M. A. Barilla and Prof. Bern Kohler* from Ohio State University (USA), X. Wang, H. Huang and Prof. Jean-Philip Lumb* from McGill University (Canada) and A. Manickoth, Dr. M. Bortorli and Prof. Lluís Blancafort* from the Institute of Computational Chemistry and Catalysis of the University of Girona. It has recently been published in the Nature Chemistry:
X. Wang, L. Kinziabulatova, M. Bortoli, A. Manickoth, M. A. Barilla, H. Huang, Ll. Blancafort, B. Kohler and J.-P. Lumb
“Indole-5,6-quinones display hallmark properties of eumelanin”
Nat. Chem. 2023, [], ASAP-
DOI: 10.1038/s41557-023-01175-4
Girona, April 11, 2023
For more info: gestor.iqcc@gmail.com