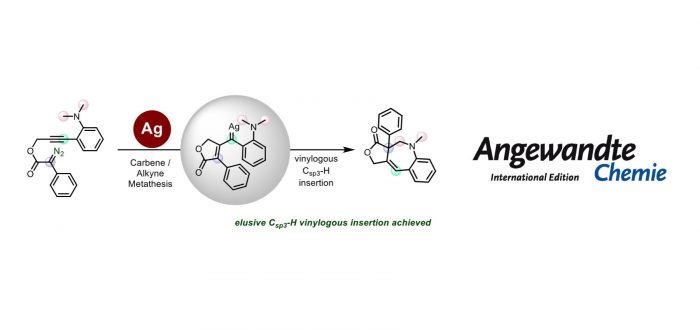
The trapping of the elusive vinylogous position of a vinyl carbene with an aliphatic Csp3-H bond has been achieved for the first time during a silver-catalyzed carbene/alkyne metathesis (CAM) process. A Tpx-containing silver complex first promotes the generation of a donor-acceptor silver carbene which triggers CAM, generating a subsequent donor-donor vinyl silver carbene species, which then undergoes a selective vinylogous C(sp3)-H bond insertion, leading to the synthesis of a new family of benzoazepines. Density functional theory (DFT) calculations unveil the reaction mechanism, which allows proposing that the C-H bond insertion reaction takes place in a stepwise manner, with the hydrogen shift being the rate determining step.
This work has been performed in collaboration with Prof. Pedro J. Pérez and Dr. Ana Caballero (CIQSO – University of Huelva, Spain), and has recently been published in Angewandte Chemie:
À. Díaz-Jiménez, R. Monreal-Corona, A. Poater, M. Álvarez, E. Borrego, P. J. Pérez, A. Caballero, A. Roglans, and A. Pla-Quintana
“Intramolecular Interception of the Remote Position of Vinylcarbene Silver Complex Intermediates by C(sp3)?H Bond Insertion”
Angew. Chem. Int. Ed., 2022, ASAP
Girona, Nov. 10, 2022
For more info: gestor.iqcc@gmail.com