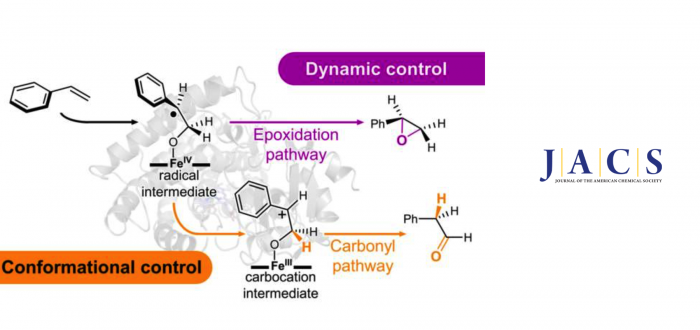
The aerobic oxidation of alkenes to carbonyls is an important and challenging transformation in synthesis. Recently, a new P450-based enzyme (aMOx) has been evolved in the laboratory to directly oxidize styrenes to their corresponding aldehydes with high activity and selectivity. The enzyme utilizes a heme-based, high-valent iron-oxo species as a catalytic oxidant that normally epoxidizes alkenes, similar to other catalysts. How the evolved aMOx enzyme suppresses the commonly preferred epoxidation and catalyzes direct carbonyl formation is currently not well understood.
In a recently published manuscript, Dr. Garcia-Borràs (PI at IQCC) and Jordi Soler (PhD student supervised by Marc), in collaboration with the experimental group of Jun.-Prof. Stephan Hammer, Emmy Noether Research Group Leader at the Bielefeld University, combined computational modelling together with mechanistic experiments to study the reaction mechanism and unravel the molecular basis behind the selectivity achieved by aMOx. Their results describe that although both pathways are energetically accessible diverging from a common covalent radical intermediate, intrinsic dynamic effects determine the strong preference for epoxidation. They discovered that aMOx overrides these intrinsic preferences by controlling the accessible conformations of the covalent radical intermediate. This disfavors epoxidation and facilitates the formation of a carbocation intermediate that generates the aldehyde product through a fast 1,2-hydride migration. Electrostatic preorganization of the enzyme active site also contributes to the stabilization of the carbocation intermediate. Computations predicted that the hydride migration is stereoselective due to the enzymatic conformational control over the intermediate species. These predictions were corroborated by experiments using deuterated styrene substrates, which proved that the hydride migration is cis- and enantioselective. Their results demonstrated that directed evolution tailored a highly specific active site that imposes strong steric control over key fleeting biocatalytic intermediates, which is essential for accessing the carbonyl forming pathway and preventing competing epoxidation.
This project part of the research program that Dr. Garcia-Borràs leads at the IQCC, which is devoted to the use of computational methods in combination with experiments to characterize and design new abiological enzymatic activities and synthetically useful biocatalysts on “Biocatalytic intermediates for the discovery and design of new enzymatic activities”. It is also a core part of the PhD thesis of Jordi Soler, PhD student at the IQCC under the supervision of Dr. Garcia-Borràs.
The computational predictions gathered in this work, were experimentally verified by the experimental group of Jun.-Prof. Stephan Hammer, Emmy Noether Research Group Leader at the Bielefeld University, in the framework of a new established collaboration between both research teams.
These results have recently been published in the Journal of the American Chemical Society:
J. Soler, S. Gergel, C. Klaus, S. C. Hammer, and M. Garcia-Borràs
“Enzymatic Control over Reactive Intermediates Enables Direct Oxidation of Alkenes to Carbonyls by a P450 Iron-Oxo Species”
J. Am. Chem. Soc. 2022, 144, 15954–15968
DOI: 10.1021/jacs.2c02567
Girona, Sep. 12, 2022
For more info: gestor.iqcc@gmail.com