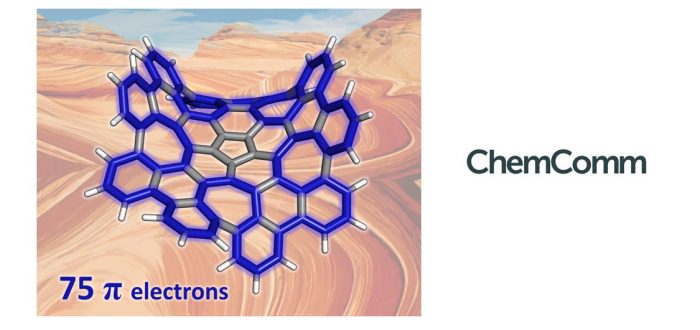
Aromaticity in macrocycles has been less studied than aromaticity in small polycyclic aromatic hydrocarbons (PAHs). In the work carried out by Dr. Álvaro Muñoz Castro from the Grupo de Química Inorgánica y Materiales Moleculares of the Universidad Autónoma de Chile and by Sílvia Escayola, Dr. Albert Poater, and Prof. Miquel Solà of the Institute of Computational Chemistry and Catalysis (IQCC) of the University of Girona, the authors analyze the most efficient circuits for pi-delocalization in a grossly warped nanographene (C80H30), containing five- and seven-membered rings inserted into a six-membered mesh. DFT calculations of different aromaticity indices (FLU, HOMA, EDDB, and ring currents) indicate that one of the two most favorable circuits for pi-electron delocalization formally has 50 pi-electrons abiding by Hückel’s rule, whereas the second one formally has 75 pi-electrons and, remarkably, it does not follow any of the known rules of aromaticity. The nanographene studied display both local aromaticity in the external six-membered rings and macrocyclic aromaticity in the 50 and 75 pi-electrons circuit. This is the first time that a pi-electronic circuit has an odd number of electrons, and also that the circuit involve cross-conjugated pathways (i.e. they do not have alternating single and double bonds).
This finding has been highlighted by Chemistry World, and was recently published in Chemical Communications:
S. Escayola, A. Poater, A. Muñoz-Castro, and M. Solà
“An unprecedented ?-electronic circuit involving an odd number of carbon atoms in a grossly warped non-planar nanographene”
Chem. Commun. 2021, ASAP-
DOI: 10.1039/D1CC00593F