alpha-Amino acids are considered a valuable class of natural products that are employed as building blocks in biological and chemical synthesis. However, only a limited number of natural amino acids are available. Nowadays, and due to their widespread application in diagnosis, proteomics, drug delivery and catalysis, there is an increasing demand for the development of procedures for the preparation of modified amino acids.
One of the most straightforward approach consists in the chemical modification of pre-existing amino acid structures via side-chain C-H functionalization. Among all the methodologies, Pd-catalyzed transformation is the most pursued methods. In these reactions, use of oxidants based on Ag+ and PhI(OAc)2, long reaction times (>12 hours) and high temperatures (>100ºC) are usually needed. Moreover, difunctionalization is often a competitive pathway.
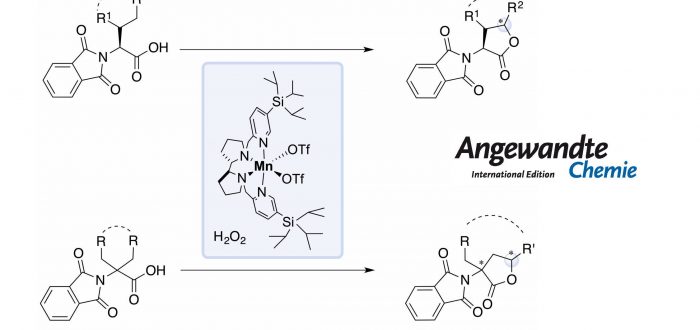
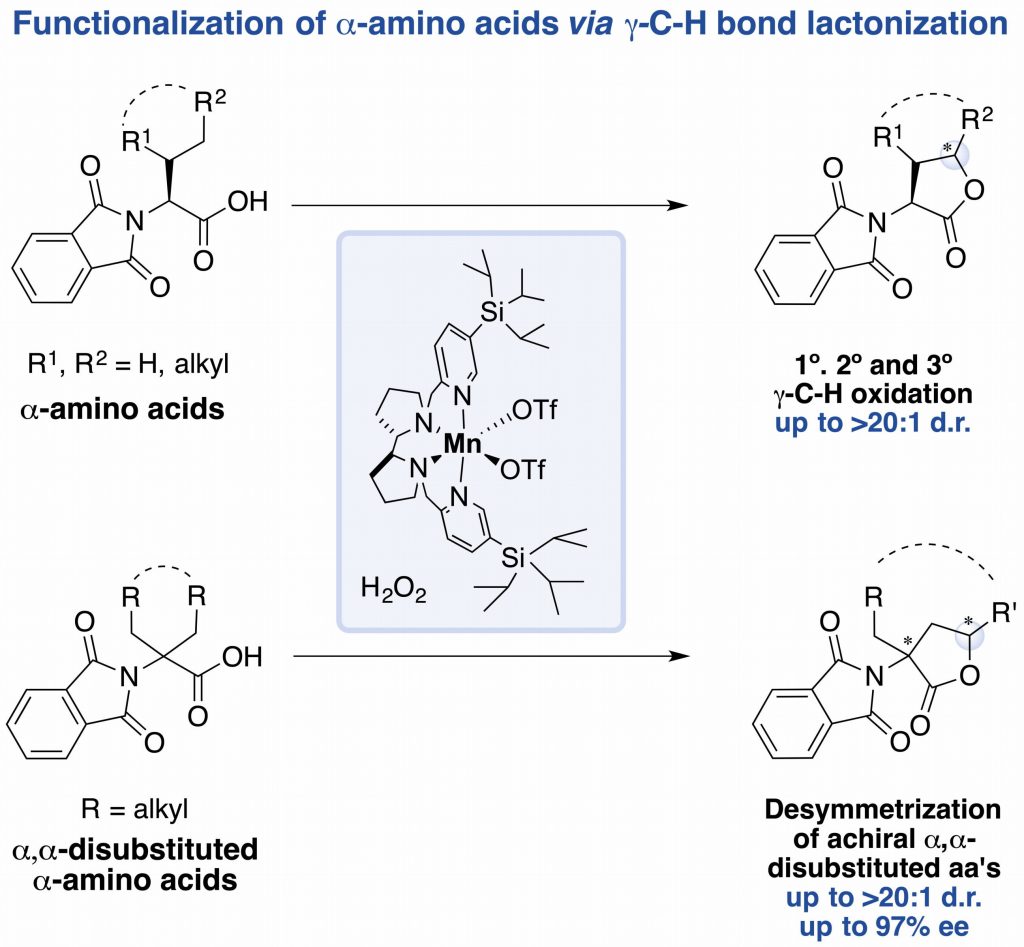
In this work, the authors show how a bioinspired manganese catalyst, in combination of H2O2 as oxidant under mild conditions (0-25ºC, under air) and short reaction times (30 minutes) provides access to modified a-amino acids via g-C-H bond lactonization. The system can efficiently target 1º, 2º and 3º g-C-H bonds of a-substituted and achiral a,a-disubstituted a-amino acids, achieving high enantioselectivities in the latter class of substrates.
This work by the group of Miquel Costas, in collaboration with Prof. Massimo Bietti from the Università degli studi di Roma “Tor Vergata” may represent an alternative methodology to the well-established organometallic procedures. The paper was published recently in Angewandte Chemie:
M. Costas, L. Vicens, and M. Bietti
“General Access to Modified alpha-Amino Acids by Bioinspired Stereoselective gamma-C-H Bond Lactonization”
Angew. Chem. Int. Ed. 2020, ASAP
DOI: 10.1002/anie.202007899
Girona, 26 November 2020
More info: gestor.iqcc@gmail.com