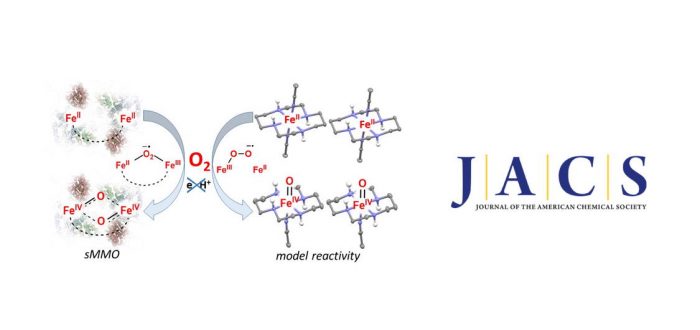
In soluble methane monooxygenase enzymes (sMMO) dioxygen (O2) is activated at a diiron(II) center to form an oxodiiron(IV) intermediate Q that performs the challenging oxidation of methane to methanol. An analogous mechanism of O2 activation at mono- or di-nuclear iron centres is rare in the synthetic chemistry. Herein, we report a mononuclear non-heme iron(II)-cyclam complex 1–trans that activates O2 to form the corresponding iron(IV)-oxo complex 2–trans via a mechanism reminiscent of the O2 activation process in sMMO. The conversion of 1–trans to 2–trans proceeds via the intermediate formation of an iron(III)-superoxide species 3, which could be trapped and spectroscopically characterized at –50°C. Surprisingly, 3 is a stronger oxygen atom transfer (OAT) agent than 2–trans; 3 performs OAT to 1–trans or PPh3 to yield 2–trans quantitatively. Furthermore, 2–trans oxidizes the aromatic C-H bonds of 2,6-di-tert-butylphenol, which, together with the strong OAT ability of 3 represent new domains of oxoiron(IV) and superoxoiron(III) reactivities. The paper was published this week in Journal of the American Chemical Society:
D. Kass, T. Corona, K. Warm, B. Braun-Cula, U. Kuhlmann, E. Bill, S. Mebs, M. Swart, H. Dau, M. Haumann, P. Hildebrandt, and K. Ray
“Stoichiometric formation of an oxoiron(IV) complex by a soluble methane monooxygenase type activation of O2 at an iron(II)-cyclam centre”
J. Am. Chem. Soc. 2020, ASAP [abstract]
DOI: 10.1021/jacs.9b13756