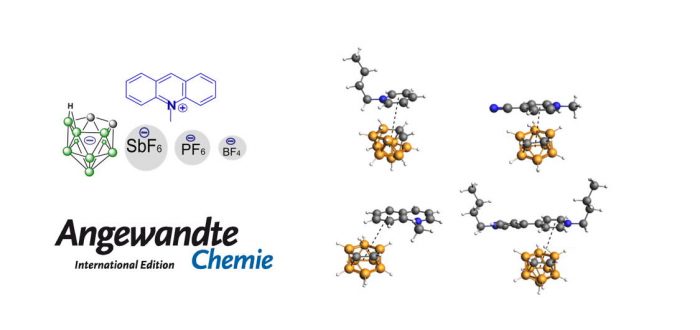
Exploration and comprehension of chemical bonding is one of the central tasks in chemistry. Here, a non-covalent interaction, a nido-cage···? bond, is discovered in a collaborative work between Deshuang Du and Prof. Yan from Nanjing University and Prof. Jordi Poater at the University of Barcelona (ex-IQCC) and Prof. Miquel Solà of IQCC (Univ. Girona). The new interaction found is between the boron cluster C2B9H12? and several aromatic ? systems. The X-ray diffraction studies indicate that the nido-cage···? bonding presents parallel-displaced or T-shaped geometries. The theoretical calculations confirms that this nido-cage···? bond shares a similar nature to the conventional anion···? or ?···? bonds found in classical aromatic ring systems. Besides, such a nido-cage···? interaction induces variable photophysical properties such as aggregation-induced emission and aggregation-caused quenching in one molecule. This work offers an overall understanding towards the boron cluster-based non-covalent bond and opens a door to investigate its properties. The paper was recently published in Angewandte Chemie – International Edition:
H. Yan, D. Tu, J. Poater, and M. Solà
“nido-Cage···? Bond: A Non-covalent Interaction between Boron Clusters and Aromatic Rings and Its Applications”
Angew. Chem. Int. Ed. 2020, ASAP [abstract]
DOI: 10.1002/anie.201915290