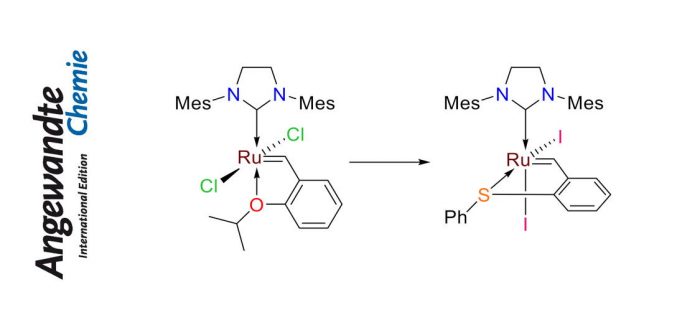
Olefin metathesis seems to be a complex chemical reaction, but if we say that leads to the formation of polymers, i.e. plastics, it is really simple and it is valid in any item that currently we have in our hands. This field of olefin metathesis is mature, after the Nobel Prize in 2005 of Chauvin, Schrock and Grubbs, but still here with two simple modifications we have been able to get novel catalysts that lead to amazing selectivity. In collaboration with the Ben-Gurion University of the Negev, the IQCC team led by Albert Poater shows that cis-diiodo sulfur chelated ruthenium benzylidenes do not react with strained cycloalkenes and internal olefins, but can effectively catalyze metathesis reactions of terminal dienes. Surprisingly, internal olefins may partake in olefin metathesis reactions once the ruthenium methylidene intermediate is generated. This unexpected behavior allows the facile formation of strained cis-cyclooctene by the RCM reaction of 1,9-undecadiene. Moreover, cis-1,4-polybutadiene may be transformed to small cyclic molecules, including its smallest precursor, 1,5-cyclooctadiene, by the use of this novel sequence. Norbornenes, including the reactive DCPD, remain unscathed even in the presence of terminal olefin substrates; being too bulky to approach the diiodo ruthenium methylidene. Once in silico calculations confirmed the promising different behavior of those novel catalysts, the impressively latent catalyst was activated experimentally by addition of an external chloride source, unveiling a novel method for controlled polymerization of DCPD. The paper was published in Angewandte Chemie International Edition.
N.B. Nechmad, R. Phatake, E. Ivry, A. Poater, and N.G. Lemcoff
“Unprecedented Selectivity of Ruthenium Iodide Benzylidenes in Olefin Metathesis Reactions”
Angew. Chem. Int. Ed. 2020, ASAP [abstract]
DOI: 10.1002/anie.201914667