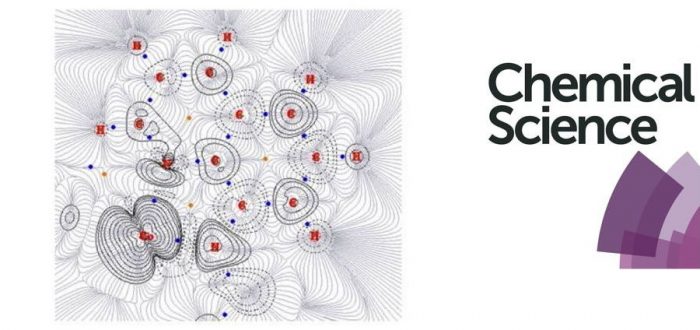
Previously, an unexpected Co-catalysed remote C-H nitration of 8-aminoquinolinamide compounds was developed. This report provided a novel reactivity for Co and was assumed to proceed through the mechanistic pathway already known for analogous Cu-catalysed remote couplings of the same substrates. In order to shed light into this intriguing, and previously unobserved reactivity for Co, a thorough computational study has now been performed, which has allowed for a full understanding of the operative mechanism. This study demonstrates that the Co-catalysed remote coupling does not occur through the previously proposed Single Electron Transfer (SET) mechanism, but actually operates through a High-Spin Induced Remote Radical Coupling mechanism, through a key intermediate with significant proportion of spin density at the 5- and 7-positions of the aminoquinoline ring. Additionally, new experimental data provides expansion of the synthetic utility of the original nitration procedure towards 1-naphthylpicolinamide which unexpectedly appears to operate via a subtly different mechanism despite having a similar chelate environment
The paper was published this week in Chemical Science
M. Chu, O. Planas, A. Company, X. Ribas, A. Hamilton, and C. J. Whiteoak.
“Unravelling the Mechanism of Cobalt-Catalysed Remote C-H Nitration of 8-Aminoquinolinamides and Expansion of Substrate Scope Towards 1-naphthylpicolinamide”
Chem. Sci. 2019, ASAP [abstract]
DOI: 10.1039/C9SC05076K