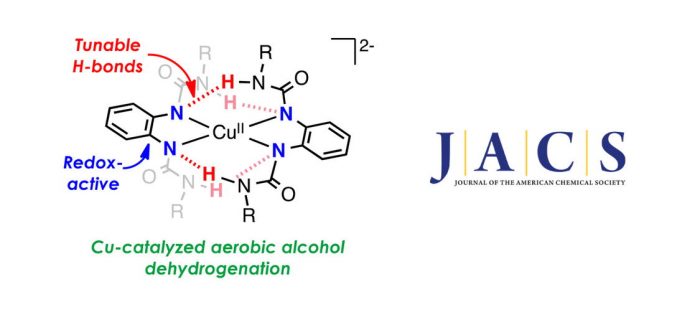
Today, Marcel Swart, Isaac Garcia-Bosch and co-workers report on catalytic aerobic oxidation of alcohols by copper complexes bearing redox-active ligands with tunable H-bonding groups. They describe the structure, spectroscopy, and reactivity of a family of copper complexes bearing bidentate redox-active ligands that contain H-bonding donor groups. Single-crystal X-ray crystallography shows that these tetracoordinate complexes are stabilized by intramolecular H-bonding interactions between the two ligand scaffolds. Interestingly, the Cu complexes undergo multiple reversible oxidation–reduction processes associated with the metal ion (CuI, CuII, CuIII) and/or the o-phenyldiamido ligand (L2–, L•–, L). Moreover, some of the CuIIcomplexes catalyze the aerobic oxidation of alcohols to aldehydes (or ketones) at room temperature. Their extensive mechanistic analysis suggests that the dehydrogenation of alcohols occurs via an unusual reaction pathway for galactose oxidase model systems, in which O2reduction occurs concurrently with substrate oxidation.
K. Rajabimoghadam, Y. Darwish, U. Bashir, D. Pitman, S. Eichelberger, M.A. Siegler, M. Swart, and I. Garcia-Bosch
“Catalytic Aerobic Oxidation of Alcohols by Copper Complexes Bearing Redox-Active Ligands with Tunable H-Bonding Groups”
J. Am. Chem. Soc. 2018, 140, 16625-16634 [abstract]
DOI: 10.1021/jacs.8b08748