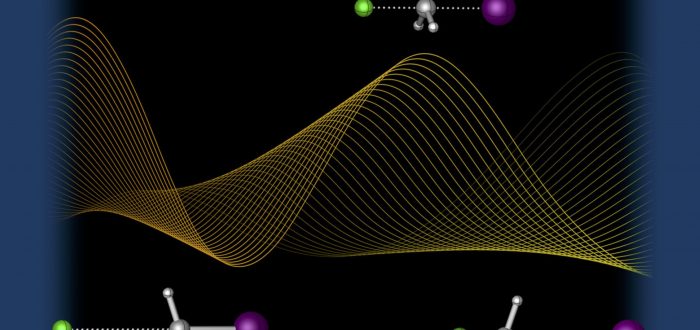
Today a mini-review on bimolecular nucleophilic substitution (SN2) reactions was published in ChemPhysChem, by Trevor Hamlin, Marcel Swart and Matthias Bickelhaupt. It describes the dependence of the energy profile on the nucleophile, leaving group, central atom, substituents, and solvent:
The reaction potential energy surface (PES), and thus the mechanism of bimolecular nucleophilic substitution (SN2), depends profoundly on the nature of the nucleophile and leaving group, but also on the central, electrophilic atom, its substituents, as well as on the medium in which the reaction takes place. Here, we provide an overview of recent studies and demonstrate how changes in any one of the aforementioned factors affect the SN2 mechanism. One of the most striking effects is the transition from a double?well to a single?well PES when the central atom is changed from a second?period (e.?g. carbon) to a higher?period element (e.g, silicon, germanium). Variations in nucleophilicity, leaving group ability, and bulky substituents around a second?row element central atom can then be exploited to change the single?well PES back into a double?well. Reversely, these variations can also be used to produce a single?well PES for second?period elements, for example, a stable pentavalent carbon species.
Apart from the mini-review itself, it is also accompanied by the cover of the journal.

T.A. Hamlin, M. Swart, and F.M. Bickelhaupt
“Nucleophilic Substitution (SN2): Dependence on Nucleophile, Leaving Group, Central Atom, Substituents, and Solvent”
ChemPhysChem 2018, 19, 1315-1330 [abstract]
Mini-review DOI: 10.1002/cphc.201701363
Cover DOI: 10.1002/cphc.201800352