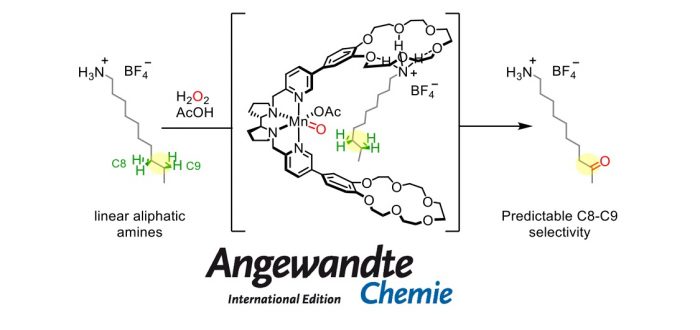
Site-selective C-H functionalization of aliphatic alkyl chains is a longstanding challenge in oxidation catalysis, given the comparable relative reactivity of the different methylenes. A supramolecular, bioinspired approach is described to address this challenge. A Mn complex able to catalyze C(sp3)-H hydroxylation with H2O2 is equipped with 18-benzocrown-6 ether receptors that bind ammonium substrates via hydrogen bonding. Reversible pre-association of protonated primary aliphatic amines with the crown ether selectively exposes remote positions (C8 and C9) to the oxidizing unit, resulting in a site-selective oxidation. Remarkably, such control of selectivity retains its efficiency for a whole series of linear amines, overriding the intrinsic reactivity of C?H bonds, no matter the chain length.
The paper was published today in Angewandte Chemie-International Edition:
G. Olivo, G. Farinelli, A. Barbieri, O. Lanzalunga, S. Di?Stefano, and M. Costas
“Supramolecular Recognition Allows Remote, Site-Selective C-H Oxidation of Methylenic Sites in Linear Amines”
Angew. Chem. Int. Ed. 2017, 56, 16347-16351 [abstract]
DOI: 10.1002/anie.201709280