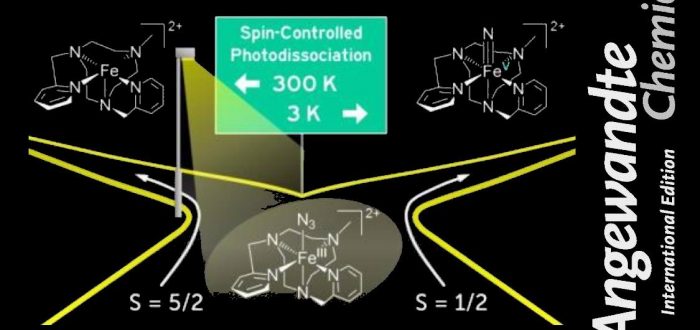
We report the generation of iron(V) nitride complexes, which are targets of biomimetic chemistry. Temperature-dependent ion spectroscopy shows that this reaction is governed by the spin state population of their iron(III) azide precursors and can be tuned by temperature. The complex [(MePy2TACN)Fe(N3)]2+ exists as a mixture of sextet and doublet spin states at 300 K, whereas only the doublet state is populated at 3 K. Photofragmentation of the sextet state complex leads to the reduction of the iron centre. The doublet state complex photodissociates to the desired iron(V) nitride complex. To generalize these findings, we show results for complexes with cyclam-based ligands.
The results were published today in Angewandte Chemie – International Edition (www.dx.doi.org/10.1002/anie.201707420):
E. Andris, R. Navratil, J. Jasik, G. Sabenya, M. Costas, M. Srnec, and J. Roithová
“Spin State-Controlled Photodissociation of Iron(III) Azide to Iron(V) Nitride Complex”
Angew. Chem. Int. Ed. 2017, [], ASAP- [abstract]
DOI: 10.1002/anie.201707420