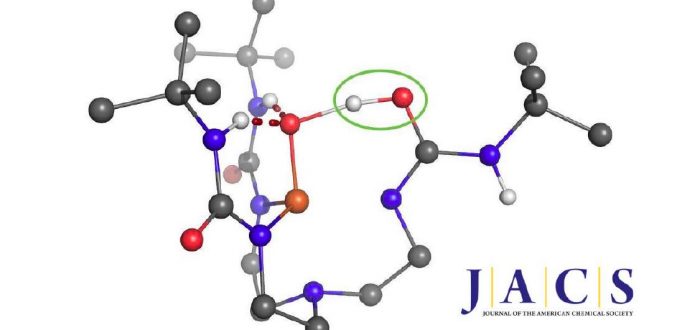
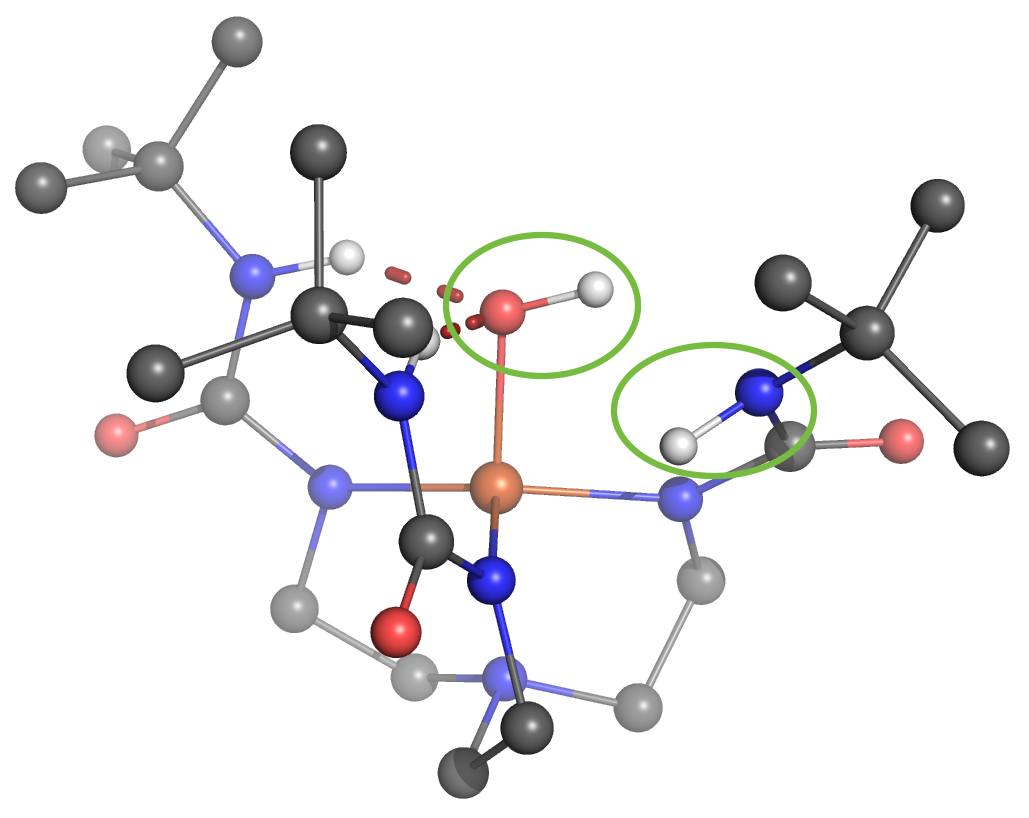
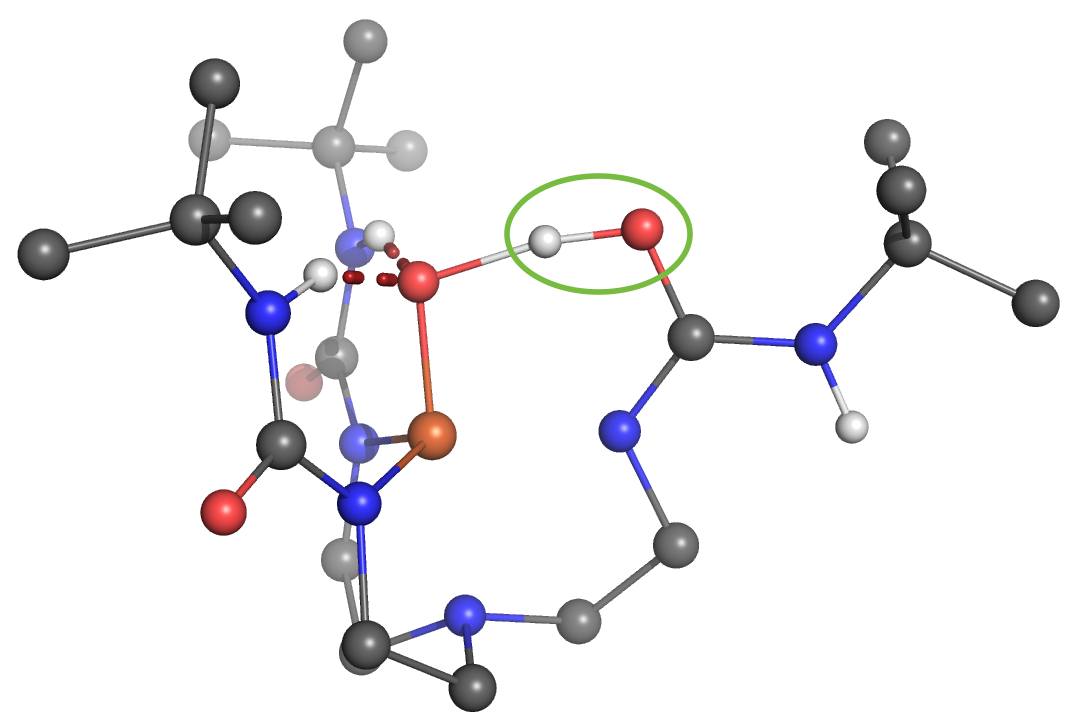
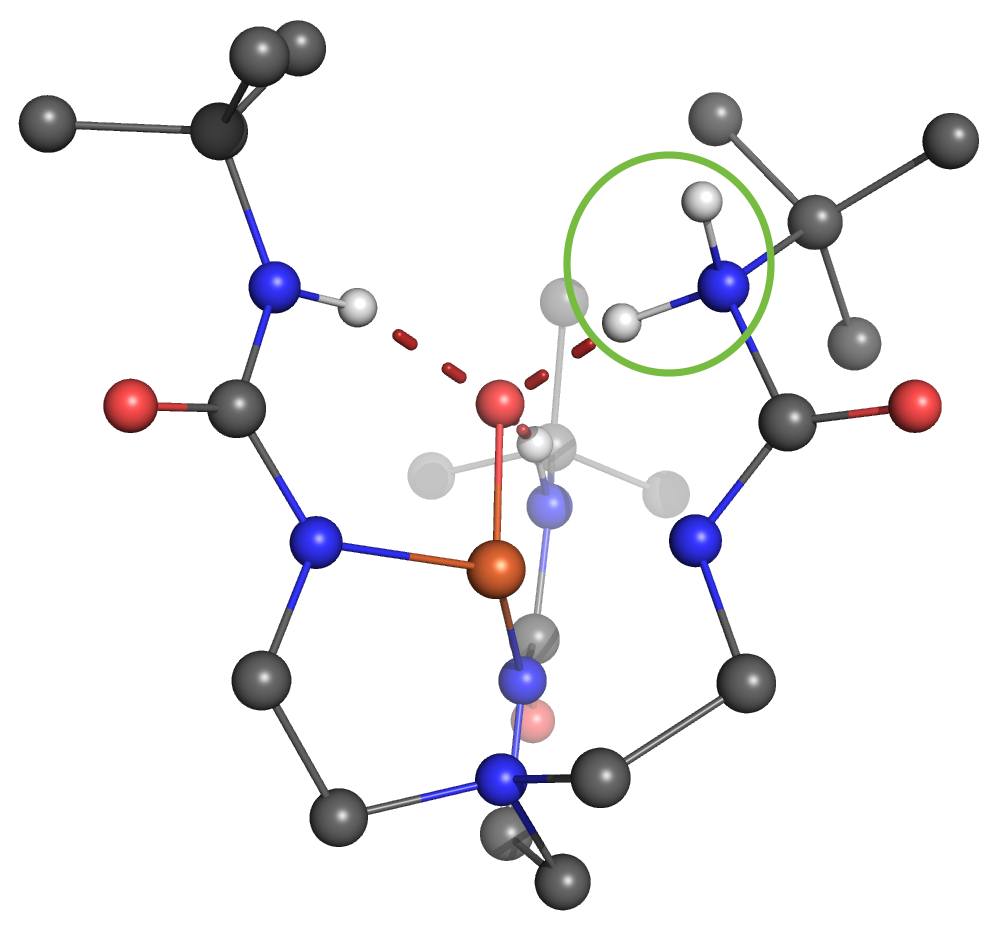
One of the Plenary Speakers of the Girona Seminar, Prof. Borovik (UC Irvine), asked Prof. Swart for his help in understanding where the proton goes in a high-valent “Fe(IV)-hydroxo” complex. Together with Adrià Romero, they were able to figure out that there were four viable possibilities where the proton would be either at the “oxo”-oxygen, at the neighboring urea unit (directly at the nitrogen, or on the urea oxygen after rotation), or even located at a carbonyl that is further away.
 |
 |
 |
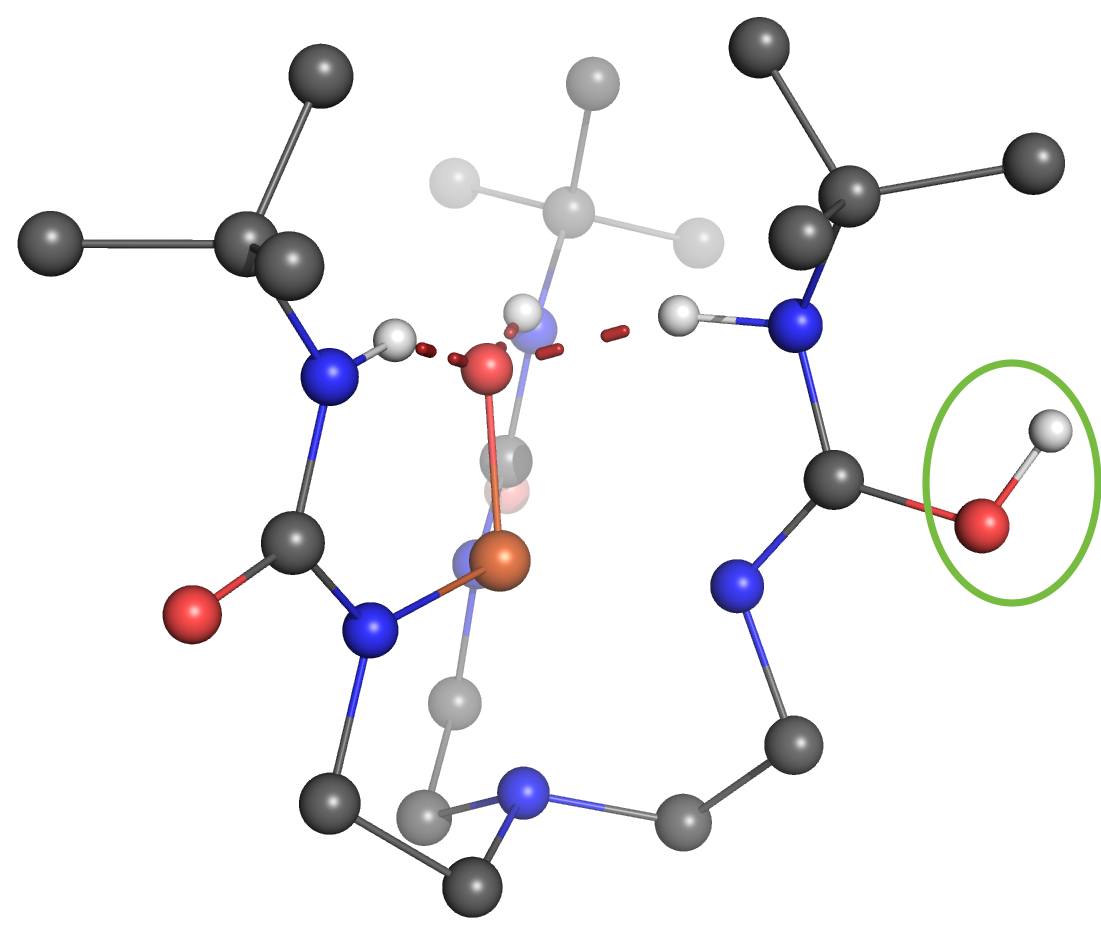 |
Using the same computational setup that Prof. Swart used for a validation study on 16 Fe(III)/Fe(IV)-oxo/hydroxo/peroxo complexes (Chem. Commun. 2013, 49, 6650), they were able to figure out the most likely position for the proton, and could verify this by computing the corresponding Mössbauer parameters. These findings, together with X-ray Absorption Spectroscopy (XAS) and Nuclear Resonance Vibrational Spectroscopy (NRVS) clearly indicate that protonation of the Fe(IV)-oxo complex most likely occurs on the tripodal ligand. It suggests that similar protonated high-valent Fe-oxo species may occur in the active sites of proteins. This finding further argues for caution when assigning unverified high-valent Fe-OH species to mechanisms. The results were published today in the Journal of the American Chemical Society:
E.A. Hill, A.C. Weitz, E. Onderko, A. Romero-Rivera, Y. Guo, M. Swart, E.L. Bominaar, M.T. Green, M.P. Hendrich, D.C. Lacy, and A.S. Borovik
“Reactivity of an FeIV -Oxo Complex with Protons and Oxidants”
J. Am. Chem. Soc. 2016, 138, 13143-13146
[abstract]
DOI: 10.1021/jacs.6b07633