- sec.iqcc@udg.edu
- +34 972 41 83 57
Pla-Quintana, Anna
Transition Metals in Organic Synthesis.
Contact info:
Dr. Anna Pla-Quintana
anna.plaq@udg.edu
Tel. (+34) 972 41 82 75
Website

Selected publications
Àlex Díaz-Jiménez, Roger Monreal-Corona, Albert Poater, María Álvarez, Elena Borrego, Pedro J. Pérez, Ana Caballero, Anna Roglans, Anna Pla-Quintana
Intramolecular Interception of the Remote Position of Vinylcarbene Silver Complex Intermediates by C(sp3)-H Bond Insertion
Angew. Chem. Int. Ed. 2023, 62, e202215163
DOI: 10.1002/anie.202215163
Hot paper
Jordi Vila, Miquel Sola?, Thierry Achard, Stephane Bellemin-Laponnaz, Anna Pla-Quintana, and Anna Roglans
Rh(I) Complexes with Hemilabile Thioether-Functionalized NHC Ligands as Catalysts for [2 + 2 + 2] Cycloaddition of 1,5-Bisallenes
ACS Catal. 2023, 13, 3201?3210
DOI: 10.1021/acscatal.2c05790
Àlex Díaz-Jiménez, Stuart C.D. Kennington, Anna Roglans, and Anna Pla-Quintana
Copper(I) Iodide Catalyzed [3 + 3] Annulation of Iodonium Ylides with Pyridinium 1,4-Zwitterionic Thiolates for the Synthesis of 1,4?Oxathiin Scaffolds
Org.Lett. 2023, 25, 4830?4834
DOI: 10.1021/acs.orglett.3c01538
Albert Artigas, Cristina Castanyer, Nil Roig, Agustí Lledó, Miquel Solà, Anna Pla-Quintana, and Anna Roglans
Synthesis of Fused Dihydroazepine Derivatives of Fullerenes by a Rh-Catalyzed Cascade Process
Adv. Synth. Catal. 2021, 363, 3835– 3844
DOI: 10.1002/adsc.202100644
Jordi Vila, Roger Vinardell, Miquel Solà, Anna Pla-Quintana, and Anna Roglans
A Rh(I)-Catalyzed Cascade Cyclization of 1,5-Bisallenes and Alkynes for the Formation of cis-3,4-Arylvinyl Pyrrolidines and Cyclopentanes
Adv. Synth. Catal. 2022, 364, 206 – 217
DOI: 10.1002/adsc.202100934
Dr. Anna Pla-Quintana
Anna Pla Quintana (Banyoles, 1978) studied Chemistry at the University of Girona (1996 – 2000) and she carried out her PhD studies in the Department of Chemistry at the same university under the supervision of Dr. Anna Roglans. During her doctoral studies, she performed a three-month research-stay at the Queen’s University of Kingston (Canada) under the supervision of Prof. Victor Snieckus.
In October 2005 Anna joined the group of Prof. Jean-Pierre Majoral at the Laboratoire de Chimie de Coordination (Toulouse, France) as a postdoctoral researcher within the Fundación Ramon Areces Fellowship program. In October 2007 she returned to the University of Girona in the group of Prof. Anna Roglans as a lecturer and got a permanent position in 2009.
Anna currently leads a research team at IQCC working on the development of transition-metal catalyzed cycloaddition and cyclization reactions, with a special emphasis on the mechanistic studies with the developed procedures.
Research overview
The use of alkenes and allenes as highly versatile substrates in cyclizations to create chiral polycyclic compounds
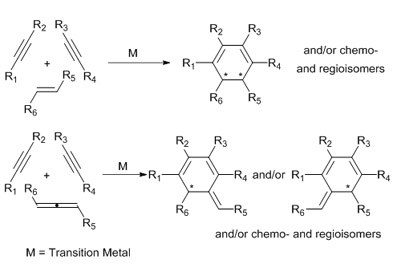
The involvement of alkenes and allenes in transition-metal calalyzed [2+2+2] cycloaddition reactions has the advantage of allowing for the eventual introduction of stereogenic centres in the newly formed six-membered ring and of affording complex polycyclic scaffolds. Our work is focused on the use of properly substituted alkenes, which are more reluctant to react than alkynes, that allow the synthesis of chiral cyclohexadienes and their further functionalization by Diels-Alder reaction. We are also investigating the use of allenes, unsaturated substrates that are more reactive than alkenes and that deliver cycloadducts with an exocyclic double bond that can subsequently be manipulated. One of the main aims in our research is to control the chemoselectivity and regioselectivity of these processes by determining the factors that govern them both experimentally and theoretically.
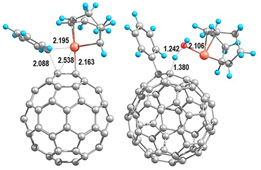
The functionalisation of fullerenes by cycloaddition reactions to provide a variety of potential molecular materials for biological, optical and electronic devices
Fullerenes are a class of molecule made up of carbon atoms with an unusual hybridization (sp2,3) exhibiting a chemical reactivity similar to that of electron-deficient olefins. [60]Fullerene, in particular, has received considerable attention due to its interesting spherical structures and unique physical and chemical properties. The functionalization of [60]fullerene with different functional groups provides a variety of potential molecular materials for biological, optical, and electronic devices. Our work is focused on exploring the versatility of rhodium complexes in the cycloaddition of pi-unsaturations to C60 as a tool to functionalize these molecules in a straightforward manner.
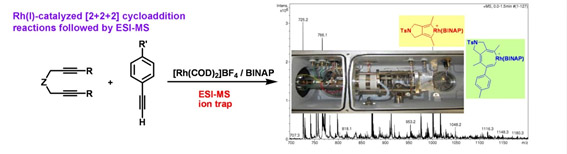
Mechanistic studies of transition-metal catalyzed reactions through electrospray ionization mass spectrometry (ESI-MS)
In order to effectively control and improve the catalytic reactions, it is necessary to understand the mechanistic details of the process. One of the difficulties faced in these investigations is that the low concentrations and transient nature of most of the intermediates involved make their identification difficult. Therefore, techniques that allow the direct monitoring of these reactive intermediates are of great interest. One of our research lines is to use electrospray ionization mass spectrometry (ESI-MS) and subsequent MS/MS analysis to obtain detailed data by trapping and identifying short-lived intermediates in transition-metal catalyzed [2+2+2] cycloaddition reactions. In addition to observing the molecular mass of ions for many reactive intermediates, this technique reveals structural information through characteristic fragmentation patterns by collision-induced dissociation. All these data help us to more fully understand the course of cycloadditions and allow us to improve these reactions.
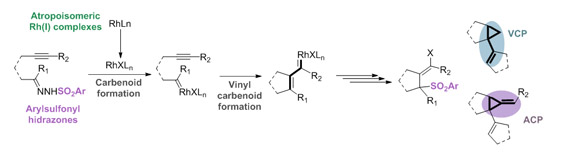
Methodological studies of cyclizations through rhodium carbenoid intermediates generated from tosylhydrazones
The vast majority of syntheses rely on step-by-step transformations. This is an inefficient, time-consuming strategy that would be greatly improved by the implementation of effective cascade processes. Of the many intermediates that can initiate or participate in cascade processes, metal-carbenoids are an excellent option due to their versatility and high reactivity. Rhodium(II) dimers have emerged as the most versatile class of complexes for single-step carbenoid mediated reactions. The transition-metal carbenoids that are required for these reactions are mostly prepared from nitrogen extrusion of diazo compounds that have non-negligible security and toxicity risks. Furthermore, the methodology is limited just to carbenoids with certain electronic properties. This research line is aimed at preparing rhodium carbenoids making use of rhodium(I) complexes to decompose arylsulfonylhydrazones, a carbenoid source that is much safer and easier to handle and which is amenable to all types of carbenoids. Rhodium(I) complexes with atropoisomeric ligands are chosen to exploit their versatility and stereoselectivity in cycloaddition reactions, nucleophilic substitutions and rearrangements. This combination is selected to trigger cascade reactions that allow the straightforward enantioselective synthesis of (poly)cyclic compounds from readily available linear starting materials.
People
Principal Investigator
Staff and Postdocs

Stuart Kennington
Postdoc (Margarita Salas)
Supervisor:- A. Pla-Quintana

Albert Artigas
Postdoc
Supervisor:- M. Solà
- A. Pla-Quintana
- A. Roglans

Anna Roglans
Full Professor
PhD and MACMoM students

Àlex Díaz
PhD Student (FPU)
Supervisor:- A. Pla-Quintana
- A. Roglans

Cristina Castanyer
PhD Student (FPI)
Supervisor:- M. Solà
- A. Pla-Quintana
- A. Roglans

Elias Antony Romero
PhD student (FI)
Supervisor:- A. Pla Quintana
- A. Roglans

Marc Rubio
PhD student (DI)
Supervisor:- A. Pla Quintana

Roger Monreal
PhD Student (FPU)
Supervisor:- A. Pla-Quintana
- A. Poater
Funding
MICIU Projects. Proyectos I+D.
Researcher: Prof. Miquel Solà and Dr. Anna Pla-Quintana
Reference: PID2020-113711GB-I00
Funding: 211.750 €
Period: 2020 – 2023
Researcher: Dr. Anna Pla-Quintana and Dr. Miquel Costas
Reference: RED2022-134074-T
Funding: 20.390 €
Period: 2023-2024
AGAUR
Researcher: Dr. Anna Pla-Quintana and Dr. Joaquim Tarres
Reference: 2022 DI 095
Funding: 33.960 €
Period: 20/02/2023 – 19/02/2026
REQ
Researcher: Dr. Stuart Kennington
Reference: REQ2021_A
Funding: 70.700 €
Period: 1/4/2022 – 31/3/2024
SGR
Researcher: Anna Roglans
Reference: 2021 SGR 00623
Funding: 60.000 €
Period: 01/01/2022 – 31/12/2024
News
New MSCA postdoctoral project for Albert Artigas
On April 4th, Dr. Albert Artigas started his MSCA post-docotral project entitled singlet
International Day of Women and Girls in Science at IQCC 2024
This coming Sunday (11th of February) is the International day of Women and
30 years IQC(C) celebration
Last 20th April took place the 30 years IQC(C) celebration. This wonderful moment
Summer scholarships for UdG students (beques d’estiu 2023)
The Institute of Computational Chemistry and Catalysis (IQCC) of the University of Girona





